By Marnie Gelbart and Nadine Vincenten
 Direct-to-consumer (DTC) genetic testing has entered our world with a big splash and opened the flood gates of genetic information. For over a decade, we have been out talking with people from all walks of life and listening to their stories. Whether we are speaking with scientists or non-scientists, we hear excitement, concerns, ambivalence – sometimes all three at the same time – and not surprisingly, many many questions as people try to make sense of it all.
Direct-to-consumer (DTC) genetic testing has entered our world with a big splash and opened the flood gates of genetic information. For over a decade, we have been out talking with people from all walks of life and listening to their stories. Whether we are speaking with scientists or non-scientists, we hear excitement, concerns, ambivalence – sometimes all three at the same time – and not surprisingly, many many questions as people try to make sense of it all.
Susan Domchek, executive director of the Basser Center for BRCA, recalls counseling a patient with a family history of breast, ovarian, and colon cancer. This patient had taken a DTC genetic test that looked at her BRCA genes, and the results led her to conclude that she was not at risk for the cancers that had burdened her family. However, the patient did not realize that the test only looked at 3 of the over 1,000 BRCA variants linked to an increased cancer risk. And because the test did not look at other genes implicated in cancer, the physician recognized that it may have underestimated her patient’s risk. What if the patient had seen a doctor who did not understand the limitations of the test? Might she have avoided taking potentially life-saving precautions?
Whereas DTC genetic testing gave this patient a false sense of security, misinterpretation of a genetic test can also lead someone to wrongly believe that they have a disease. Because genetic research has predominantly included people of European descent, people from other populations can be more vulnerable to misinterpretation of their genetic data. For some, this has led to the emotional stress of a misdiagnosis as well as unnecessary medical tests and further economic harms. Furthermore, for people of African, Indigenous, and Latinx ancestry, the mere mention of genetics often calls up the history of eugenics and forced sterilization. In this new era of genetics, how can society avoid the mistakes of the past so that everyone can benefit from advances in genetics without the fear of unethical treatment?
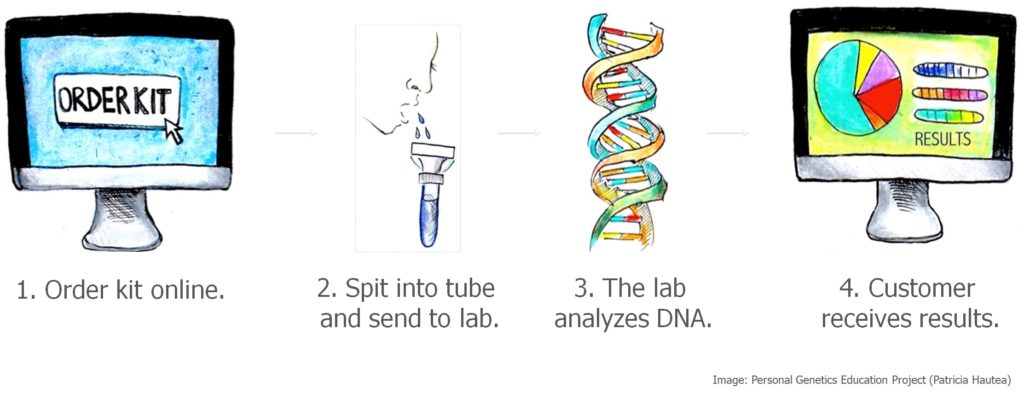
On top of the complexities of interpreting genetic data, unanticipated uses of DTC genetic data bring issues of privacy to the forefront. In 2018, many people were surprised to find that law enforcement had used a not-for-profit DNA ancestry database, called GEDmatch, to identify the suspect in a case that had gone unsolved for more than 20 years. So-called “genetic genealogy” has now become a tool that has reignited investigations in dozens of cold cases with one company, Family Tree DNA, as an active participant. However, public outcry over privacy and consent has led some consumer genetic companies to re-evaluate their policies. How can people reap the benefits they want from their genetic data without being exposed to unanticipated and potentially unwelcome consequences?
 As society grapples with the influx of genetic information, the pace of innovation in genetics continues to ramp up. With the advent of CRISPR and related genome editing technologies, it is now possible not only to read a person’s DNA, but also to change it. This is a source of hope for families struggling with genetic diseases, and there is some reason for cautious optimism as CRISPR-based therapies begin to enter clinical trials for conditions such as sickle cell disease and cancer. Given existing health disparities, how can policymakers ensure that new therapies will be equally available to all?
As society grapples with the influx of genetic information, the pace of innovation in genetics continues to ramp up. With the advent of CRISPR and related genome editing technologies, it is now possible not only to read a person’s DNA, but also to change it. This is a source of hope for families struggling with genetic diseases, and there is some reason for cautious optimism as CRISPR-based therapies begin to enter clinical trials for conditions such as sickle cell disease and cancer. Given existing health disparities, how can policymakers ensure that new therapies will be equally available to all?
These same genome editing technologies can also make changes to a person’s DNA that could be inherited by future generations. In November 2018, this line was crossed when a researcher in China announced the birth of twins whose DNA had been edited using CRISPR. There was an immediate outcry about the ethical and safety concerns, and yet there will likely be a market for so-called germline genome editing. Some people have called for a moratorium, and legislators are considering how to proceed. Are there any morally permissible applications of germline editing? Who gets to decide?
As genetics increasingly enters US courtrooms, lawyers are at the forefront of dealing with these complicated questions. And we imagine that many lawyers likely steered clear of extra science courses in college, just as most scientists likely know little about law. This is why our group, the Personal Genetics Education Project (pgEd), is committed to working with lawyers and lawmakers to help bridge this gap.
Based in the Department of Genetics at Harvard Medical School, pgEd is a team of scientists, social scientists, educators, and community organizers committed to raising awareness and dialogue about personal genetics across all communities.
We have many public-facing initiatives, including partnerships with schools, creators of television and film, and communities of faith. In addition, we have held six Congressional briefings to date to inform policymakers in Washington, D.C. And we are excited to be working to prepare the next generation of lawyers for this new genetic age.
This post is part of a digital symposium hosted by Bill of Health in conjunction with the Petrie-Flom Center’s 2019 Annual Conference, “Consuming Genetics: Ethical and Legal Considerations of New Technologies.”
Read the rest of the symposium!