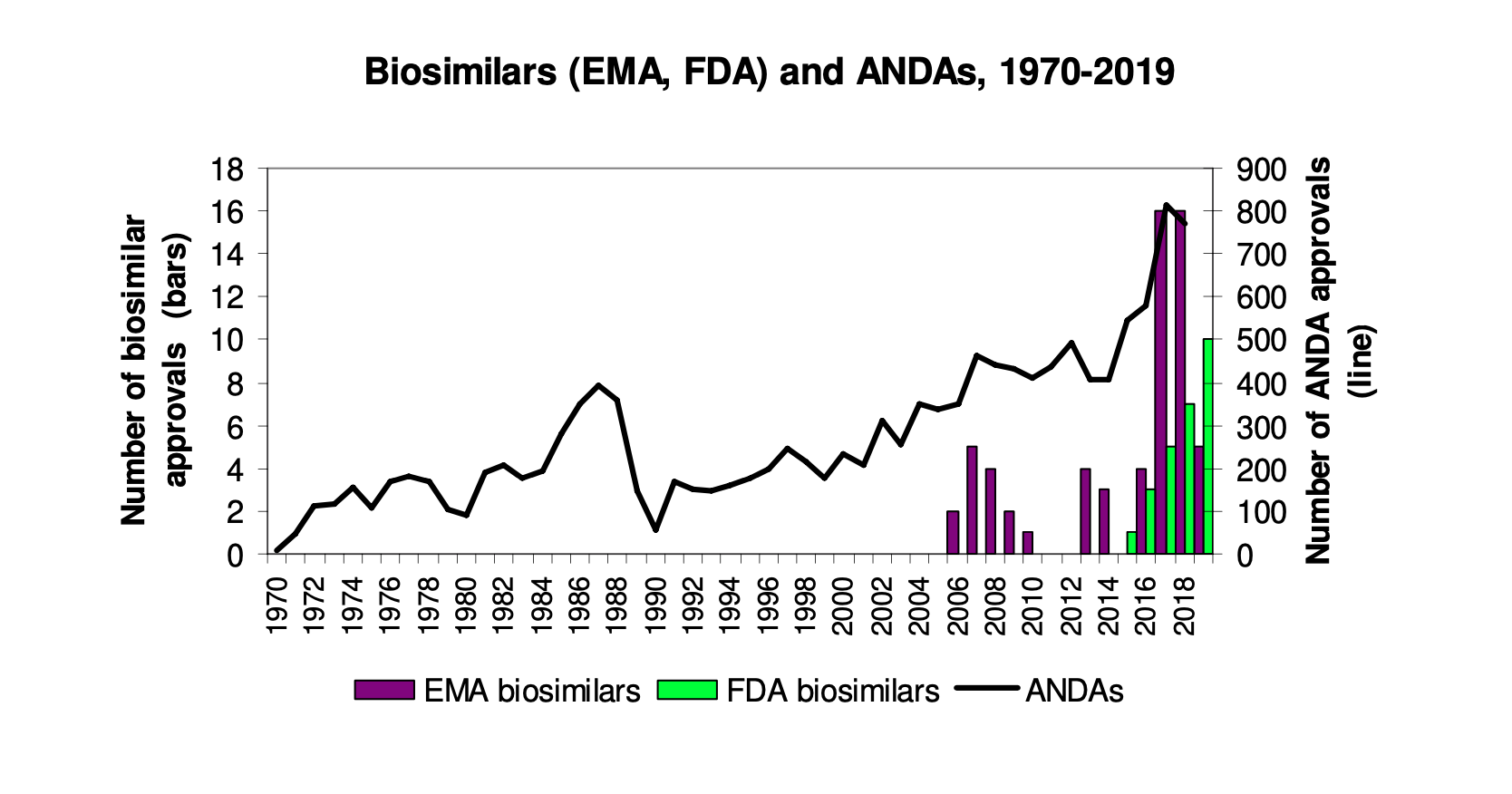
The Rise of Biosimilars: Success of the BPCIA? (Part III)
By Jonathan Darrow This is Part III in a series exploring the history, challenges, and opportunities in the regulation of biosimilars, or biologic medical products that are very similar to already approved biological medicines. Part…
The Rise of Biosimilars: Success of the BPCIA? (Part III)