AbbVie Wins First Round in Humira Antitrust Lawsuit
The purpose of the secondary patent filings was to assemble a thicket of patents, 132 in all, to prohibit competition from biosimilar companies.

The purpose of the secondary patent filings was to assemble a thicket of patents, 132 in all, to prohibit competition from biosimilar companies.

Selections feature topics ranging from political pressures facing the FDA, to the financial incentive structure for antibiotic development.
Interesting empirical studies, policy analyses, and editorials on health law and policy issues from July 2020.
While generic competition is crucial for reducing drug prices, brand-name drug manufacturers can utilize several strategies to delay such competition.

By Jonathan Darrow This is Part III in a series exploring the history, challenges, and opportunities in the regulation of biosimilars, or biologic medical products that are very similar to already approved biological medicines. Part III considers a path forward in the regulation of biologics. For Part I, click here. For Part II click here….
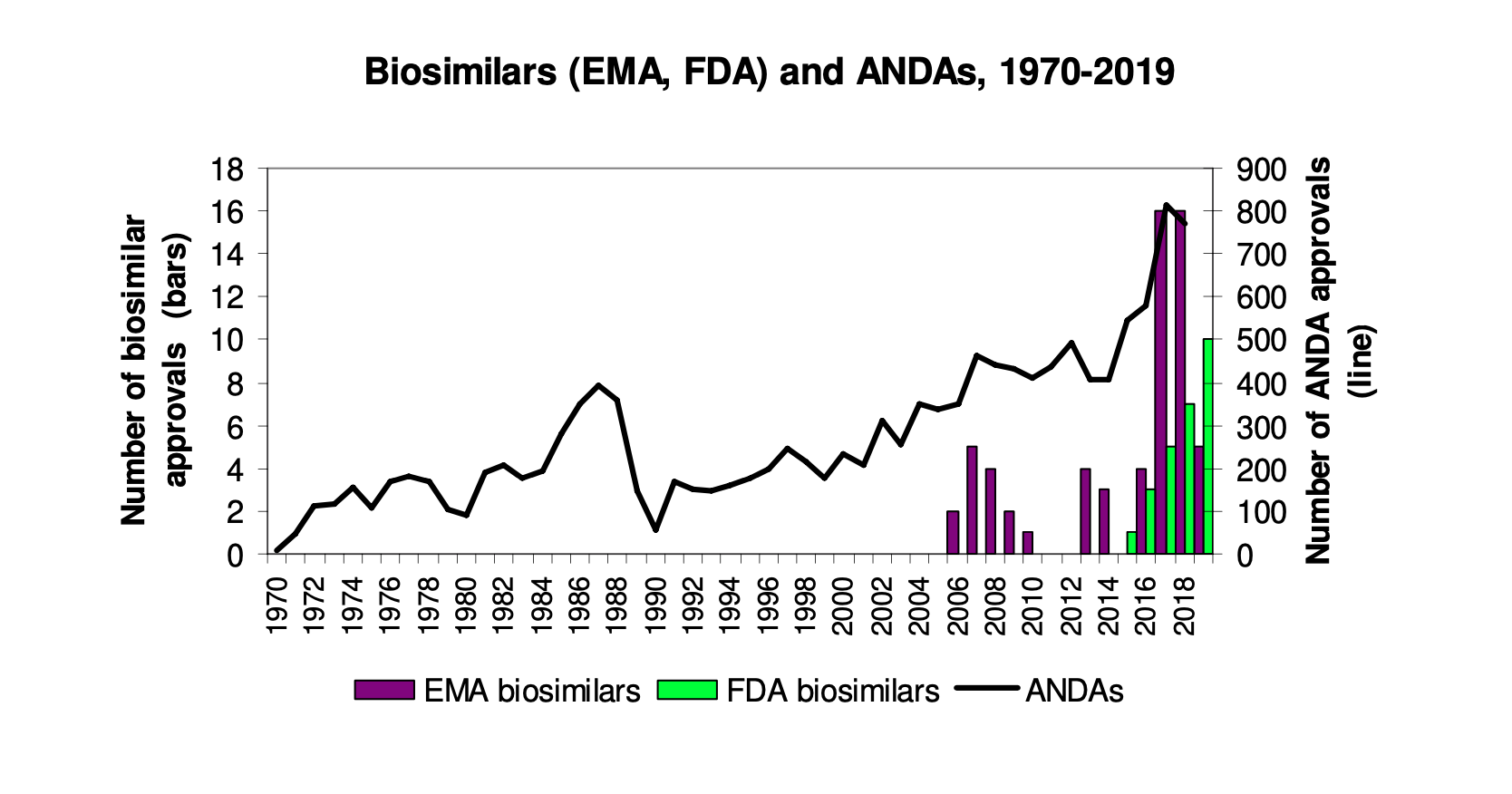
By Jonathan Darrow This is Part II in a series exploring the history, challenges, and opportunities in the regulation of biosimilars, or biologic medical products that are very similar to already approved biological medicines. Part II covers some key considerations and factors that impact the biologics market and regulation. For Part I, click here. Reference…

By Jonathan Darrow This is Part I in a series exploring the history, challenges, and opportunities in the regulation of biosimilars, or biologic medical products that are very similar to already-approved biological medicines. This Part briefly covers the history of American regulation of biologics and touches briefly on the European experience. The Biologics Price Competition…

By Ameet Sarpatwari, Charlie Lee, Frazer Tessema, and Aaron S. Kesselheim Each month, members of the Program On Regulation, Therapeutics, And Law (PORTAL) review the peer-reviewed medical literature to identify interesting empirical studies, policy analyses, and editorials on health law and policy issues relevant to current or potential future work in the Division. Below are the abstracts/summaries…

By Ameet Sarpatwari, Charlie Lee, Frazer Tessema, and Aaron S. Kesselheim Each month, members of the Program On Regulation, Therapeutics, And Law (PORTAL) review the peer-reviewed medical literature to identify interesting empirical studies, policy analyses, and editorials on health law and policy issues relevant to current or potential future work in the Division. Below are the abstracts/summaries…

By Phebe Hong On October 7th, toward the end of his health care “bill-signing marathon,” Governor Gavin Newsom signed bill AB 824, making California the first state to ban pharmaceutical “pay-for-delay” deals. The new law prohibits pay-for-delay deals, which is the practice of pharmaceutical companies giving “anything of value” to generic manufacturers to keep lower-cost…
